Pressure 1 atmosphere ~ 1 bar ~ 760 mm Hg ~ 760 torr ~ 100,000 Pa Ion gauges read in mbar i.e. 1x mbar = 1x atm. Sometimes ion gauges read. - ppt download

III Esercitaz leggi dei gas - ESERCITAZIONE N° 3 ESERCIZIO 1 Pressione atmosferica Qual è la - Studocu

Converting Pressures 760 mm Hg = 760 torr 1 atm = 760 mm Hg 1 atm = kPa 760 mm Hg = kPa Converting Pressures: Convert 740 torr to kPa ppt download
3. For a solution if pA 600 mm Hg. PB 840 mm Hg under atmospheric conditions and vapourpressure of solution is 1 atm then find(i) Composition of solution(i) Composition of vapour in

SOLVED: Convert pressure measurements from one unit to another. Given that: 1 atm = 760 mmHg = 14.7 psi = 101.325 kPa 320 mmHg into atm 30.0 atm into kPa

SOLVED: 1 atm = 760 mm Hg 760 torr = 1 atm 1 atm = 101325 Pa 101.3 J = 1 L atm 1 nm = 1 x 10^-9 m 0.08212 M^-1





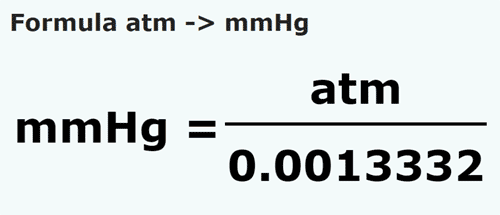





![ANSWERED] An aerosol can has a pressure of 1.86θ at... - Physical Chemistry - Kunduz ANSWERED] An aerosol can has a pressure of 1.86θ at... - Physical Chemistry - Kunduz](https://media.kunduz.com/media/sug-question/raw/76026807-1659275932.3355274.jpeg)